Published online May 27, 2022. doi: 10.4240/wjgs.v14.i5.442
Peer-review started: October 11, 2021
First decision: November 17, 2021
Revised: November 30, 2021
Accepted: April 20, 2022
Article in press: April 20, 2022
Published online: May 27, 2022
Intrahepatic cholangiocarcinoma (ICC) is the second most common primary liver cancer in humans after hepatocellular carcinoma and a rare epithelial malignancy that results in a poor prognosis. According to the Liver Cancer Study Group of Japan classification, ICC can be divided into three types: Mass-forming (MF) type, periductal-infiltrating (PI) type, and intraductal-growth type. The MF type is the most common, accounting for 57.1-83.6% of ICCs. Nevertheless, little is known about the epidemiology and treatment of MF ICC.
To examine the prognostic factors for patients with MF ICC.
We carried out a retrospective analysis of consecutive patients with MF ICC treated at the Faculty of Hepato-Pancreato-Biliary Surgery of Chinese PLA General Hospital between January 2008 and December 2018. According to the treatment received, the patients were divided into either a resection group or an exploration group.
The pooled 1-, 3-, and 5-year survival rates in the 68 patients with MF ICC were 66.5%, 36.3%, and 9.3%, respectively. Univariate analysis revealed that surgical resection (P < 0.001), nodal metastasis (P < 0.001), tumor location (P = 0.039), vascular invasion (P < 0.001), ascites (P < 0.001), and differentiation (P = 0.009) were significantly associated with the prognosis and survival of MF ICC. Multivariate analysis revealed that ascites (hazard ratio [HR] = 5.6, 95% confidence interval [CI]: 1.6-18.9, P = 0.006) and vascular invasion (HR = 2.5, 95%CI: 1.0-6.1, P = 0.045) were independent risk factors for MF ICC. The pooled 1-, 3-, and 5-year survival rates in the 19 patients of the exploration group were 5.3%, 5.3%, and 0, respectively. Among the 49 patients who underwent surgical resection, the pooled 1-, 3-, and 5-year survival rates were 93.5%, 49.7%, and 14.4%, respectively. Univariate and multivariate analyses revealed that vascular invasion (HR = 3.1, 95%CI: 1.2-8.5, P = 0.024) and nodal metastasis (HR = 3.2, 95%CI: 1.4-7.6, P = 0.008) were independent prognostic risk factors for surgical resection patients.
The prognosis of MF ICC patients is dismal, especially those with ascites or vascular invasion. Surgical resection is a key factor in improving overall survival in patients with MF ICC, and vascular invasion and lymph node metastasis affect the efficacy of surgical resection.
Core Tip: This is a single-center, large-scale retrospective study on mass-forming intrahepatic cholangiocarcinoma (MF ICC) to examine the prognostic factors for MF ICC and improve the outcomes. The study found the patients with MF ICC with ascites and vascular invasion have a poor prognosis. Surgical resection is a key factor in improving overall survival in patients with MF ICC, and patients with vascular invasion and lymph node metastasis have poor surgical results.
- Citation: Feng J, Liang B, Zhang HY, Liu Z, Jiang K, Zhao XQ. Prognostic factors for patients with mass-forming intrahepatic cholangiocarcinoma: A case series of 68 patients. World J Gastrointest Surg 2022; 14(5): 442-451
- URL: https://www.wjgnet.com/1948-9366/full/v14/i5/442.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v14.i5.442
Intrahepatic cholangiocarcinoma (ICC) refers to a malignant tumor originating from the branching epithelial cells of the intrahepatic secondary bile duct and above, with a poor prognosis[1-2]. It has been reported that both the morbidity and mortality have gradually increased in recent years[1-4]. Surgical resection is currently the only potentially curative treatment for ICC[3-5], but the cure rates and survival of patients with ICC remain very low because of the high aggressiveness of the disease[6-7]. It has been reported that many factors influence the prognosis of surgical resection[8-11].
According to the Liver Cancer Study Group of Japan classification, ICC can be divided into three types: Mass-forming (MF) type, periductal-infiltrating (PI) type, and intraductal-growth (IG) type[11]. Among them, the MF type is the most common, accounting for 57.1-83.6% of ICCs[12-14].
Nevertheless, little is known about the epidemiology and treatment of MF ICC. Therefore, the aim of the present retrospective study was to analyze prognostic factors for patients with MF ICC.
This was a retrospective analysis of consecutive patients with MF ICC treated at the Faculty of Hepato-Pancreato-Biliary Surgery of Chinese PLA General Hospital between January 2008 and December 2018. The study was approved by the Medical Ethics Committee of the Chinese PLA General Hospital.
The inclusion criteria were: (1) ≥ 18 years of age; (2) Hospitalized patients; (3) Confirmed as MF ICC by histopathological examination; and (4) No prior history of any malignancy. The exclusion criteria were: (1) Incomplete data; (2) Metastasis; (3) Hilar cholangiocarcinoma; (4) Cystadenocarcinoma; (5) PI ICC; or (6) IG ICC. The patients were divided into either a resection group or an exploration group according to the received treatment.
All cases were discussed in tumor boards before any treatment. The indications for radical hepatectomy were: (1) No distant metastases preoperatively; (2) Preoperative imaging suggesting that the tumors could be completely resected, including eventual satellite lesions; (3) Child-Pugh grade A or B; and (4) Good cardiopulmonary function and no surgical or anesthetic contraindications.
The surgical principle was to achieve R0 resection. The pattern of hepatectomy was based on residual liver function, tumour size, and tumour-vessel relationship. Anatomic resection (AR) was the priority if feasible, while non-AR (NAR) was more frequently applied if the tumour was adjacent to major vascular structure. Surgical exploration was only performed in patients with extensive metastases in the liver, abdominal wall, and omentum. Lymph node dissection of the hepatoduodenal ligament was performed for patients with lymphadenectasis found by imaging or intraoperatively. Tumor and lymph node biopsies were performed in patients undergoing surgical exploration.
General data and results of auxiliary examinations were recorded, including carbohydrate antigen 19-9 (CA19-9), hepatitis B virus (HBV), glutamic pyruvic transaminase (ALT), glutamic oxaloacetic transaminase, alkaline phosphatase, gamma-glutamyltransferase, and total bilirubin tests.
All patients were followed after surgery. Follow-up visits were performed once every 3 mo during the first year, once every 6 mo during the second and third years, and once a year later. Items checked during the follow-up visits included routine laboratory tests, tumor markers, chest roentgenogram, abdominal ultrasound, CT, and/or MRI examinations. The follow-up deadline was December 31, 2019, and the follow-up duration ranged from 1 to 82 mo, with a median duration of 13 mo.
All statistical analyses were performed using IBM SPSS Statistics ver. 21.0 (IBM Co, Armonk, NY, United States). Continuous data meeting a normal distribution are presented as the mean ± SD. Differences between the two groups were determined using independent sample t test. Continuous data not meeting a non-normal distribution are presented as the median (range). The non-parametric Mann-Whitney U test was used to determine the differences between the two groups. The chi-square test or the Fisher’s exact test was used for categorical data. Univariate Cox proportional hazard regression model analysis was used for survival data. Variables with P < 0.05 in univariate analysis were included in the multivariate Cox proportional hazard regression model. Kaplan-Meier analysis was used to calculate the survival rate. Log-rank method was used for group-wise comparison. Two-sided P values < 0.05 were considered statistically significant.
Among the 68 patients, 50 were male and 18 female, ranging from 24 to 74 years with a median age of 54. There were 40 patients with tumors in the right lobe of the liver and 28 with tumors in the left lobe of the liver. The median tumor diameter was 7.0 cm (range, 2.2-14.0). Twenty-eight (41.2%) patients had elevated CA 19-9 levels, five of whom had CA 19-9 > 1000 U/mL. Sixteen and four had concomitant hepatitis B and C viral infections, respectively. Fourteen cases were accompanied with ascites. The characteristics were similar between the two groups, except that the exploration group had higher levels of ALT (P = 0.031), higher frequencies of ascites (P < 0.001), nodal metastasis (P < 0.001), and vascular invasion (P < 0.001), and the tumors were mostly located in the left lobe (P < 0.001) (Table 1).
| Variable | All (n = 68) | Surgery (n = 49) | Exploration (n = 19) | P value |
| Age (yr) | 54.3 ± 1.4 | 52.6 ± 1.7 | 58.6 ± 2.2 | 0.435 |
| Gender, Male | 50 (73.5%) | 34 (69.4%) | 16 (84.2%) | 0.924 |
| HBV infection | 16 (23.5%) | 13(26.5%) | 3 (15.8%) | 0.997 |
| HCV infection | 4 (5.9%) | 2 (4.1%) | 2 (10.5%) | 0.314 |
| Ascites | 14 (20.6%) | 1 (2.0%) | 13(68.4%) | < 0.001 |
| Tumor size(cm) | 6.9 ± 0.3 | 6.8 ± 0.4 | 7.63 ± 0.5 | 0.495 |
| ALT (IU/L)(median) | 1.8-92.1 (26) | 1.8-92.1 (24.9) | 23-76.3 (32.1) | 0.031 |
| AST (IU/L) (median) | 9.6-74.2 (29) | 9.6-74.2 (27.3) | 18.2-61.9 (31) | 0.142 |
| ALP (U/L) (median) | 13.4-280.5 (82.8) | 13.4-280.5 (81.4) | 45.3-109.9 (85.4) | 0.149 |
| GGT (U/L) (median) | 11-325.6 (42.4) | 11-325.6 (41.1) | 28.9-104.7 (45.8) | 0.512 |
| TBIL (mg/dL) (median) | 4.2-140.0 (18) | 4.2-140 (18.1) | 4.2-42.6 (17.8) | 0.707 |
| CA19-9 (U/mL) (median) | 21-2000 (34.5) | 21-1891 (36) | 22-2000 (30) | 0.104 |
| Differentiation | 0.536 | |||
| Poor | 30 (44.1%) | 20 (40.8%) | 10 (40.052.6 | |
| Poor-moderate | 24 (35.3%) | 19 (38.8%) | 5 (26.3%) | |
| Moderate | 14 (20.6%) | 10 (20.4%) | 4 (21.1%) | |
| Nodal metastasis | 33 (48.5%) | 14 (28.6%) | 19 (100.0%) | < 0.001 |
| Tumor location | < 0.001 | |||
| Left lobe | 28 (41.2%) | 11 (22.4%) | 17 (89.5%) | |
| Right lobe | 40 (58.8%) | 38 (77.6%) | 2 (10.5%) | |
| Vascular invasion | 31 (45.6%) | 13 (26.5%) | 19 (100.0%) | < 0.001 |
All patients were discharged successfully from the hospital. During follow-up, 48 patients died and 20 survived. Survival time ranged from 1 to 82 mo (median, 24 mo). The pooled 1-, 3-, and 5-year survival rates in the 68 patients with MF ICC were 66.5%, 36.3%, and 9.3%, respectively (Table 2). Univariate analysis revealed that surgical resection (P < 0.001), nodal metastasis (P < 0.001), tumor location (P = 0.039), vascular invasion (P < 0.001), ascites (P < 0.001), and differentiation (P = 0.009) were significantly associated with the prognosis and survival of MF ICC (Table 3). Multivariate analysis revealed that ascites (hazard ratio [HR] = 5.6, 95% confidence interval [CI]: 1.6-18.9, P = 0.006) and vascular invasion (HR = 2.5, 95%CI: 1.0-6.1, P = 0.045) were independent risk factors for MF ICC (Table 3).
| All (n = 68) | Surgery (n = 49) | Exploration (n = 19) | P value | |
| Follow-up (mo) | 1-82 | 3-82 | 1-57 | |
| Survival | < 0.001 | |||
| 1 yr | 66.5% | 93.5% | 5.3% | |
| 3 yr | 36.3% | 49.7% | 5.3% | |
| 5 yr | 9.3% | 14.4% | 0.00% |
| Variable | Patients (n) | 1 yr (%) | 3 yr (%) | 5 yr (%) | P value | HR | 95%CI | P value |
| Age (yr) | 0.278 | |||||||
| ≤ 54 | 35 | 71.8 | 39.8 | 13.5 | ||||
| >54 | 33 | 61.4 | 32.7 | 6.1 | ||||
| Gender | 0.292 | |||||||
| Male | 50 | 62.2 | 34.2 | 9.7 | ||||
| Female | 18 | 79.6 | 43.0 | 10.8 | ||||
| HBV infection | 0.327 | |||||||
| Yes | 16 | 74.0 | 24.7 | 0 | ||||
| No | 52 | 64.0 | 40.0 | 13.3 | ||||
| Ascites | < 0.001 | 5.553 | 1.628-18.941 | 0.006 | ||||
| Present | 14 | 0 | 0 | 0 | ||||
| Absent | 54 | 84.0 | 45.8 | 11.8 | ||||
| Tumor size (cm) | 0.230 | |||||||
| ≤ 7 | 41 | 64,3 | 49.0 | 10.1 | ||||
| > 7 | 27 | 70.2 | 12.5 | 6.3 | ||||
| CA 19-9 (IU/mL) | 0.881 | |||||||
| ≤ 27 | 40 | 62.7 | 36.6 | 7.8 | ||||
| > 27 | 28 | 72.3 | 34.8 | 15.5 | ||||
| Differentiation | 0.009 | 0.769 | 0.466-1.270 | 0.305 | ||||
| Poor | 30 | 56.4 | 21.7 | 0 | ||||
| Poor-moderate | 24 | 78.5 | 62.4 | 12.8 | ||||
| Moderate | 14 | 66.1 | 23.6 | 23.6 | ||||
| Nodal metastasis | < 0.001 | 2.294 | 0.983-5.353 | 0.055 | ||||
| Yes | 35 | 97.0 | 64.0 | 21.7 | ||||
| No | 33 | 37.8 | 9.1 | 0 | ||||
| Tumor location | 0.032 | 2.186 | 0.801-5.965 | 0.127 | ||||
| Left lobe | 28 | 40.9 | 28.6 | 0 | ||||
| Right lobe | 40 | 86.8 | 43.9 | 12.4 | ||||
| Vascular invasion | < 0.001 | 2.501 | 1.020-6.131 | 0.045 | ||||
| Yes | 31 | 35.5 | 9.7 | 0 | ||||
| No | 37 | 97.1 | 66.3 | 22.2 | ||||
| Group | < 0.001 | 1.619 | 0.351-7.469 | 0.537 | ||||
| Resection | 49 | 93.5 | 49.7 | 14.4 | ||||
| Exploration | 19 | 5.3 | 5.3 | 0 |
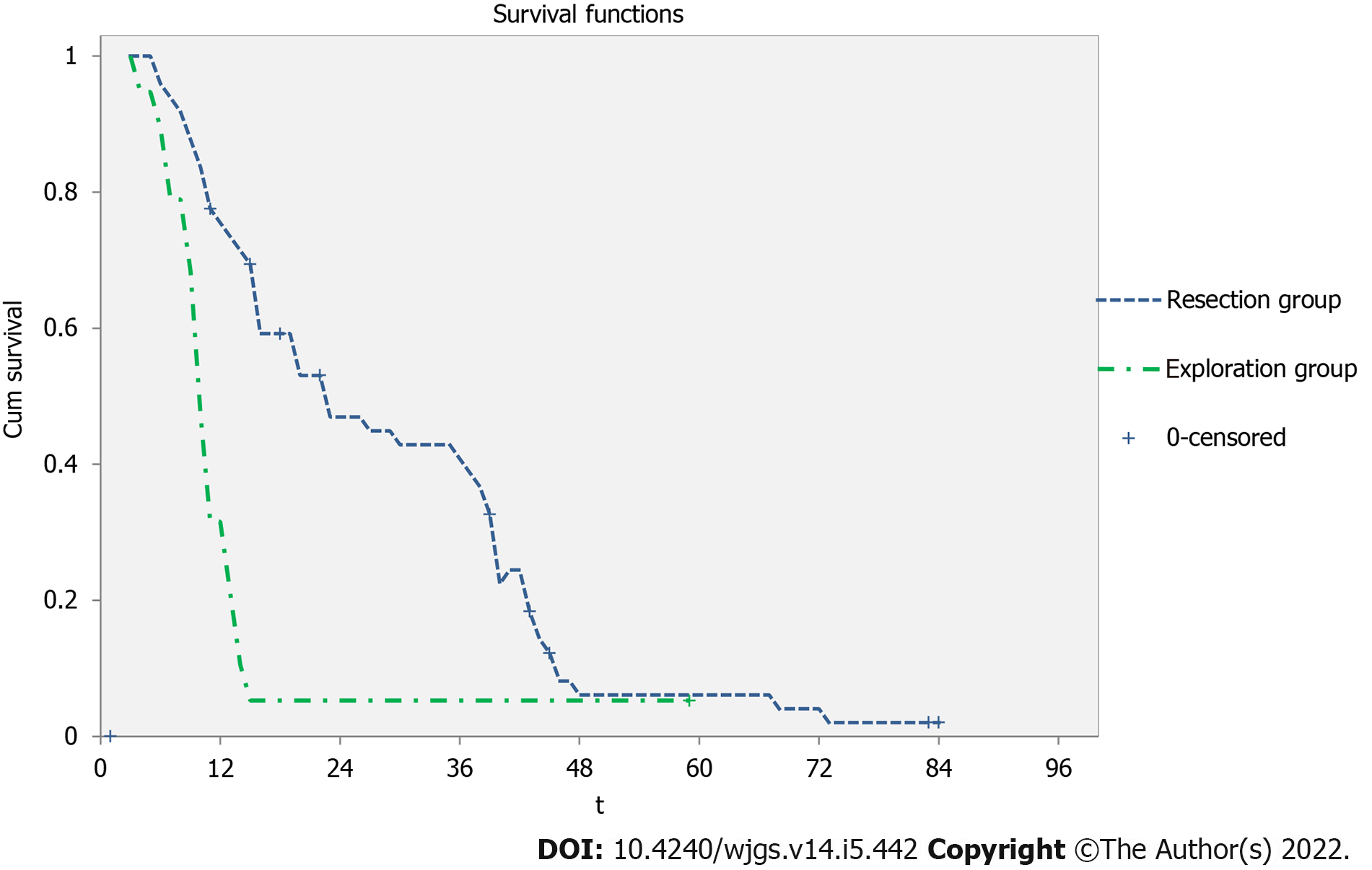
The pooled 1-, 3-, and 5-year survival rates in the 19 patients of the exploration group were 5.3%, 5.3%, and 0, respectively. Correspondingly, the pooled 1-, 3-, and 5-year survival rates in the 49 patients of the surgical resection group were 93.5%, 49.7%, and 14.4%, respectively. The survival rates of the resection group were significantly better than those of the exploration group (P < 0.001) (Figure 1). Table 4 presents the univariate and multivariate analyses of the factors associated with survival in the surgery group. Unlike the whole group of patients, univariate and multivariate analyses revealed that vascular invasion (HR = 3.1, 95%CI: 1.2-8.5, P = 0.024) and nodal metastasis (HR = 3.2, 95%CI: 1.4-7.6, P = 0.008) were independent prognostic risk factors for surgical resection patients.
| Variable | Patients (n) | 1 yr (%) | 3 yr (%) | 5 yr (%) | P value | HR | 95%CI | P value |
| Age (yr) | 0.633 | |||||||
| ≤ 54 | 27 | 92.3 | 48.6 | 21.2 | ||||
| > 54 | 22 | 95.0 | 50.7 | 9.5 | ||||
| Gender | 0.441 | |||||||
| Male | 34 | 90.9 | 48.2 | 18.1 | ||||
| Female | 15 | 100.0 | 54.0 | 13.5 | ||||
| HBV infection | 0.063 | |||||||
| Yes | 13 | 92.3 | 30.8 | 0 | ||||
| No | 36 | 94.0 | 57.1 | 22.5 | ||||
| Ascites | 0.836 | |||||||
| Present | 1 | 0 | 0 | 0 | ||||
| Absent | 48 | 93.4 | 49.6 | 14.4 | ||||
| Tumor size (cm) | 0.044 | 1.273 | 0.485-3.339 | 0.624 | ||||
| ≤ 7 | 28 | 92.9 | 69.6 | 16.9 | ||||
| > 7 | 21 | 94.1 | 33.6 | 8.4 | ||||
| CA 19-9 (IU/mL) | 0.571 | |||||||
| ≤ 27 | 26 | 96.0 | 53.9 | 12.9 | ||||
| > 27 | 23 | 90.6 | 43.7 | 19.4 | ||||
| Differentiation | 0.061 | |||||||
| Poor | 20 | 89.7 | 34.5 | 0 | ||||
| Poor-moderate | 19 | 94.7 | 73.9 | 23.9 | ||||
| Moderate | 10 | 100.0 | 35.7 | 35.7 | ||||
| Nodal metastasis | 0.001 | 3.221 | 1.364-7.610 | 0.008 | ||||
| Yes | 35 | 97.0 | 64.0 | 21.7 | ||||
| No | 14 | 85.7 | 11.9 | 0 | ||||
| Tumor location | 0.545 | |||||||
| Left lobe | 11 | 100.0 | 66.7 | 33.3 | ||||
| Right lobe | 38 | 91.4 | 46.3 | 13.0 | ||||
| Vascular invasion | < 0.001 | 3.148 | 1.160-8.544 | 0.024 | ||||
| Yes | 12 | 83.3 | 16.7 | 0 | ||||
| No | 37 | 97.1 | 66.3 | 22.2 | ||||
| Pattern of liver resection | 0.773 | |||||||
| AR resection | 23 | 96.0 | 50.6 | 11.4 | ||||
| NAR resection | 25 | 95.5 | 51.7 | 9.7 | ||||
| Resection margin(cm) | 0.361 | |||||||
| ≤ 1 | 21 | 95.2 | 40.3 | 16.1 | ||||
| > 1 | 27 | 96.0 | 57.3 | 14.6 |
Little is known about the epidemiology and treatment of MF ICC. Therefore, this study aimed to examine the prognostic factors for patients with MF ICC. The results showed that the prognosis of MF ICC patients is dismal, especially those with ascites or vascular invasion. Resectable patients have a better prognosis, and vascular invasion and lymph node metastasis affected the efficacy of surgical resection. It is reported that the morbidity of ICC in males is 40-63.5%[14,16-18], and the age at diagnosis is mainly in the 6th decade of life, but ranges from 21 to 86 years[17-20]. Among the 68 cases in the current study, 50 were males, accounting for 73.5% of the patients, which was higher than that reported in the literature. The age of onset was 24-74 years with a median age of 54 years, which was consistent with literature reports but could still be a little younger than that in the literature. This discrepancy could be due to a number of reasons including genetics, environment, and methods of detection.
Many previous studies showed that HBV and hepatitis C virus (HCV) infections were associated with the occurrence of ICC. It has been reported that the rate of HBV infection ranges from 3.9% to 28.8% in ICC patients, and the rate of HCV infection ranges from 0.6% to 16.5%[20-22]. In the present study, the infection rates of HBV and HCV were 23.5% and 5.9%, respectively, which were similar to those reported in the literature. Currently, the relationship between HBV and ICC prognosis is still controversial. Pan et al[23] reported that the 1- and 3-year overall survival rates of patients with HBV infection was higher than that of patients without (67.6% and 47.2% vs 43.8% and 18.4%, respectively). Ahn et al[24] reported that HBV infection itself was not regarded as an independent prognostic factor. Tao et al[25] described that 1-, 3-, and 5-year cumulative survival rates of HBsAg-positive ICC patients are significantly lower than those of HBV-negative ICC patients. The present study found that there was no significant difference in survival between patients with HBV infection and those without. Nevertheless, among the 68 patients, the 5-year survival was 0 in patients with HBV infection, while it was 13.3% in those without HBV infection. In the surgery group, the 5-year survival was 0 in patients with HBV infection, while it was 22.5% in patients without HBV infection. These rates raise the question of the impact of HBV infection on the survival of ICC patients and further study is needed to investigate this point.
Surgical resection is the most important factor for long-term survival of ICC patients. In this study, the 5-year survival rate was 14.4% for patients in the resection group, while it was 0% for patients in the exploration group. The surgical approach required tumor-free surgical margins, i.e., R0 resection. The literature has reported that the R0 resection rate of ICC ranges from 24.1% to 92.8%[10,26], but the relationship between margins and survival is still controversial in patients with ICC. Bagante et al[13] deemed that patients with positive margins had a poor prognosis. Tang et al[16] reported that the prognosis in patients with margins > 1 cm was better than that of patients with margins ≤ 1 cm, while Bartsch et al[10] showed that the margin width was not related to prognosis. Other studies reported that no significant difference in survival was observed between patients with R0 resection and patients with R1 resection[7,27,28]. In the present study, the resection rate was 72.1% (49/68), and all resections were R0. Whether the margins were > 1 cm or not was not related to survival. Furthermore, there was no significant difference in 1-, 3-, and 5-year survival rates between AR and NAR resection (96.0%, 50.6%, and 11.4% vs 95.5%, 51.7%, and 9.7%, respectively). These results suggest that the objective is to achieve R0 no mater using AR or NAR resection. A number of studies have indicated that patients with positive lymph nodes have a poor prognosis[11,13,17,18]. Bagante et al[13] showed that the 5-year survival rate in patients with positive lymph nodes was 9.4%, while in patients with negative lymph nodes, it was 45.5%. In the present study, the 5-year survival rate in patients of the resection group and with positive lymph nodes was 0%, compared with 21.7%, in patients with negative lymph nodes. Lymph node metastasis could be an important prognostic factor for ICC. Nevertheless, there is still no definite conclusion as to whether resection of positive lymph nodes can extend survival or not[17,18,29,30].
Previous studies showed that vascular invasion was an important factor affecting the prognosis of ICC[27,31,32] . Our results revealed that the 3- and 5-year survival rates in the resection group with vascular invasion were 16.7% and 0%, respectively, compared with 66.3% and 22.2%, respectively, in patients without. The survival rate in patients without vascular invasion was higher than that of patients with vascular invasion. The multivariate analysis revealed that vascular invasion was an independent prognostic factor in patients with ICC.
In the present study, there was no significant difference in survival for left and right lobe tumors in the resection group. However, in the whole group of 68 patients, the resection rate of tumor in the right lobe was 95.0% (38/40), and that in the left lobe was 39.3% (11/28), indicating that the resection rate of tumors in the left lobe was low. Survival analysis also suggested that the survival rate was low for patients with tumors in the left lobe, which may be because tumors in the left lobe are more prone to metastasis through the ligament of the liver and stomach. In addition, we also noted that tumors in the left lobe could metastasize from the round ligament of the liver and sickle ligament of the liver to the abdominal wall. Nevertheless, further study is necessary for confirmation.
Data revealed that 25%-40% of the tumors with metastasis could not be dissected by surgical exploration for ICC patients whose tumors are considered to be removable before surgery. Therefore, laparoscopic examination should be performed before operation for patients with multicentric lesions, high CA19-9, suspected vascular infiltration, or peritoneal carcinomatosis[4]. In the present study, 19 patients (27.9%) underwent surgical exploration. Among the 40 cases with tumors in the right lobe of the liver, 5% (n = 2) underwent surgical exploration, while 60.7% (n = 17) underwent surgical exploration among the 28 patients with tumors in the left lobe of the liver, suggesting that the exploration rate was high for tumors in the left lobe of the liver. Among the 14 cases with preoperative ascites, there were 13 cases with abdominal metastasis and peritoneal metastasis. Therefore, we believe that routine laparoscopic exploration should be performed before operation for patients with tumors in the left lobe of the liver or with ascites in order to avoid meaningless laparotomy.
The present study is not without limitations. This was a retrospective, single-center study with a small sample size. In addition, it was limited to Chinese patients. Thus, the results should be validated using multicenter studies.
The prognosis of MF ICC patients is dismal, especially those with ascites or vascular invasion. Surgical resection is a key factor in improving overall survival in patients with MF ICC, and vascular invasion and lymph node metastasis affect the efficacy of surgical resection.
The mass-forming (MF) type is the most common intrahepatic cholangiocarcinoma (ICC), accounting for 57.1%-83.6% of ICCs. Nevertheless, little is known about the epidemiology and treatment of MF ICC.
To improve the outcomes of ICC.
To examine the prognostic factors for patients with MF ICC.
We carried out a retrospective analysis of consecutive patients with MF ICC. The patients were divided into either a resection group or an exploration group according to the treatment received.
The pooled 1-, 3-, and 5-year survival rates in the 68 patients with MF ICC were 66.5%, 36.3%, and 9.3%, respectively. Univariate analysis revealed that surgical resection (P < 0.001), nodal metastasis (P < 0.001), tumor location (P = 0.039), vascular invasion (P < 0.001), ascites (P < 0.001), and differentiation (P = 0.009) were significantly associated with the prognosis and survival of MF ICC. Multivariate analysis revealed that ascites (hazard ratio [HR] = 5.6, 95% confidence interval [CI]: 1.6-18.9, P = 0.006) and vascular invasion (HR = 2.5, 95%CI: 1.0-6.1, P = 0.045) were independent risk factors for MF ICC. The pooled 1-, 3-, and 5-year survival rates in the 19 patients of the exploration group were 5.3%, 5.3%, and 0, respectively. Among the 49 patients who underwent surgical resection, the pooled 1-, 3-, and 5-year survival rates were 93.5%, 49.7%, and 14.4%, respectively. Univariate and multivariate analyses revealed that vascular invasion (HR = 3.1, 95%CI: 1.2-8.5, P = 0.024) and nodal metastasis (HR = 3.2, 95%CI: 1.4-7.6, P = 0.008) were independent prognostic risk factors for surgical resection patients.
The prognosis of MF ICC patients is dismal, especially those with ascites or vascular invasion. Surgical resection is a key factor in improving overall survival in patients with MF ICC, and vascular invasion and lymph node metastasis affect the efficacy of surgical resection.
Surgical resection is a key factor in improving overall survival in patients with MF ICC, and vascular invasion and lymph node metastasis affect the efficacy of surgical resection.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Andersen JB, Denmark; Jusakul A, Thailand; Pandya S, United States S-Editor: Wang LL L-Editor: Wang TQ P-Editor: Wang LL
| 1. | Bridgewater J, Galle PR, Khan SA, Llovet JM, Park JW, Patel T, Pawlik TM, Gores GJ. Guidelines for the diagnosis and management of intrahepatic cholangiocarcinoma. J Hepatol. 2014;60:1268-1289. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 862] [Cited by in F6Publishing: 945] [Article Influence: 94.5] [Reference Citation Analysis (0)] |
| 2. | Moeini A, Sia D, Bardeesy N, Mazzaferro V, Llovet JM. Molecular Pathogenesis and Targeted Therapies for Intrahepatic Cholangiocarcinoma. Clin Cancer Res. 2016;22:291-300. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 141] [Cited by in F6Publishing: 154] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 3. | Ransome E, Tong L, Espinosa J, Chou J, Somnay V, Munene G. Trends in surgery and disparities in receipt of surgery for intrahepatic cholangiocarcinoma in the US: 2005-2014. J Gastrointest Oncol. 2019;10:339-347. [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 4. | Weber SM, Ribero D, O'Reilly EM, Kokudo N, Miyazaki M, Pawlik TM. Intrahepatic cholangiocarcinoma: expert consensus statement. HPB (Oxford). 2015;17:669-680. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 250] [Cited by in F6Publishing: 298] [Article Influence: 33.1] [Reference Citation Analysis (0)] |
| 5. | Cillo U, Fondevila C, Donadon M, Gringeri E, Mocchegiani F, Schlitt HJ, Ijzermans JNM, Vivarelli M, Zieniewicz K, Olde Damink SWM, Groot Koerkamp B. Surgery for cholangiocarcinoma. Liver Int. 2019;39 Suppl 1:143-155. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 109] [Cited by in F6Publishing: 169] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 6. | Bartsch F, Hahn F, Müller L, Baumgart J, Hoppe-Lotichius M, Kloeckner R, Lang H. Relevance of suspicious lymph nodes in preoperative imaging for resectability, recurrence and survival of intrahepatic cholangiocarcinoma. BMC Surg. 2020;20:75. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 7. | Luvira V, Eurboonyanun Ch, Bhudhisawasdi V, Pugkhem A, Pairojkul Ch, Luvira V, Sathitkarnmanee E, Somsap K, Kamsa-ard S. Patterns of Recurrence after Resection of Mass-Forming Type Intrahepatic Cholangiocarcinomas. Asian Pac J Cancer Prev. 2016;17:4735-4739. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 8] [Reference Citation Analysis (0)] |
| 8. | Buettner S, Galjart B, van Vugt JLA, Bagante F, Alexandrescu S, Marques HP, Lamelas J. Performance of prognostic scores and staging systems in predicting long-term survival outcomes after surgery for intrahepatic cholangiocarcinoma. J Surg Oncol. 2017;116:1085-1095. [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 9. | Conci S, Ruzzenente A, Viganò L, Ercolani G, Fontana A, Bagante F, Bertuzzo F, Dore A, Pinna AD, Torzilli G, Iacono C, Guglielmi A. Patterns of Distribution of Hepatic Nodules (Single, Satellites or Multifocal) in Intrahepatic Cholangiocarcinoma: Prognostic Impact After Surgery. Ann Surg Oncol. 2018;25:3719-3727. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 10. | Bartsch F, Baumgart J, Hoppe-Lotichius M, Straub BK, Heinrich S, Lang H. Intrahepatic cholangiocarcinoma - influence of resection margin and tumor distance to the liver capsule on survival. BMC Surg. 2020;20:61. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 11. | Ma CH, Hwang DW, Song KB, Kim SC, Shin SH, Lee JH. Prognostic factors predicting survival rate over 10 years of patients with intrahepatic cholangiocarcinoma after hepatic resection. Ann Surg Treat Res. 2020;98:116-123. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 12. | Uno M, Shimada K, Yamamoto Y, Nara S, Esaki M, Sakamoto Y, Kosuge T, Ojima H. Periductal infiltrating type of intrahepatic cholangiocarcinoma: a rare macroscopic type without any apparent mass. Surg Today. 2012;42:1189-1194. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 13. | Bagante F, Weiss M, Alexandrescu S, Marques HP, Aldrighetti L, Maithel SK, Pulitano C, Bauer TW, Shen F, Poultsides GA, Soubrane O, Martel G, Koerkamp BG, Guglielmi A, Itaru E, Pawlik TM. Long-term outcomes of patients with intraductal growth sub-type of intrahepatic cholangiocarcinoma. HPB (Oxford). 2018;20:1189-1197. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 14. | Dover LL, Jacob R, Wang TN, Richardson JH, Redden DT, Li P, DuBay DA. Improved Postoperative Survival for Intraductal-Growth Subtype of Intrahepatic Cholangiocarcinoma. Am Surg. 2016;82:1133-1139. [PubMed] [Cited in This Article: ] |
| 15. | Ohira M, Yoshizumi T, Yugawa K, Kosai-Fujimoto Y, Inokuchi S, Motomura T, Mano Y, Toshima T, Itoh S, Harada N, Ikegami T, Soejima Y, Taketomi A, Mori M. Association of inflammatory biomarkers with long-term outcomes after curative surgery for mass-forming intrahepatic cholangiocarcinoma. Surg Today. 2020;50:379-388. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 16. | Tang H, Lu W, Li B, Meng X, Dong J. Influence of surgical margins on overall survival after resection of intrahepatic cholangiocarcinoma: A meta-analysis. Medicine (Baltimore). 2016;95:e4621. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 17. | Navarro JG, Lee JH, Kang I, Rho SY, Choi GH, Han DH. Prognostic significance of and risk prediction model for lymph node metastasis in resectable intrahepatic cholangiocarcinoma: do all require lymph node dissection? HPB (Oxford). 2020;8:S1365. [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 18. | Asaoka T, Kobayashi S, Hanaki T, Iwagami Y, Tomimaru Y, Akita H, et al Clinical significance of preoperative CA199 and lymph node metastasis in intrahepatic cholangiocarcinoma. Surg Today. 2020;. [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 19. | Li Y, Wang H, Li D, Hu J, Zhou D, Li Q, Jiang X, Zhou H, Hu H. Occult hepatitis B virus infection in Chinese cryptogenic intrahepatic cholangiocarcinoma patient population. J Clin Gastroenterol. 2014;48:878-882. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 20. | De Rose AM, Cucchetti A, Clemente G, Ardito F, Giovannini I, Ercolani G, Giuliante F, Pinna AD, Nuzzo G. Prognostic significance of tumor doubling time in mass-forming type cholangiocarcinoma. J Gastrointest Surg. 2013;17:739-747. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 21. | Reddy SK, Hyder O, Marsh JW, Sotiropoulos GC, Paul A, Alexandrescu S, Marques H, Pulitano C, Barroso E, Aldrighetti L, Geller DA, Sempoux C, Herlea V, Popescu I, Anders R, Rubbia-Brandt L, Gigot JF, Mentha G, Pawlik TM. Prevalence of nonalcoholic steatohepatitis among patients with resectable intrahepatic cholangiocarcinoma. J Gastrointest Surg. 2013;17:748-755. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 22. | Wang JJ, Li H, Li JX, Xu L, Wu H, Zeng Y. Preoperative gamma-glutamyltransferase to lymphocyte ratio predicts long-term outcomes in intrahepatic cholangiocarcinoma patients following hepatic resection. World J Gastroenterol. 2020;26:1501-1512. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 11] [Cited by in F6Publishing: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 23. | Pan QX, Su ZJ, Zhang JH, Wang CR, Ke SY. Glasgow Prognostic Score predicts prognosis of intrahepatic cholangiocarcinoma. Mol Clin Oncol. 2017;6:566-574. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 24. | Ahn CS, Hwang S, Lee YJ, Kim KH, Moon DB, Ha TY, Song GW, Lee SG. Prognostic impact of hepatitis B virus infection in patients with intrahepatic cholangiocarcinoma. ANZ J Surg. 2018;88:212-217. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 25. | Tao LY, He XD, Xiu DR. Hepatitis B virus is associated with the clinical features and survival rate of patients with intrahepatic cholangiocarcinoma. Clin Res Hepatol Gastroenterol. 2016;40:682-687. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 26. | Li MX, Bi XY, Li ZY, Huang Z, Han Y, Zhao JJ, Zhao H, Cai JQ. Impaction of surgical margin status on the survival outcome after surgical resection of intrahepatic cholangiocarcinoma: a systematic review and meta-analysis. J Surg Res. 2016;203:163-173. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 27. | Kinoshita M, Kanazawa A, Takemura S, Tanaka S, Kodai S, Shinkawa H, Shimizu S, Murata A, Nishio K, Hamano G, Ito T, Tsukamoto T, Kubo S. Indications for laparoscopic liver resection of mass-forming intrahepatic cholangiocarcinoma. Asian J Endosc Surg. 2020;13:46-58. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 28. | Bektas H, Yeyrek C, Kleine M, Vondran FW, Timrott K, Schweitzer N, Vogel A, Jäger MD, Schrem H, Klempnauer J, Kousoulas L. Surgical treatment for intrahepatic cholangiocarcinoma in Europe: a single center experience. J Hepatobiliary Pancreat Sci. 2015;22:131-137. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 29. | Morine Y, Shimada M. The value of systematic lymph node dissection for intrahepatic cholangiocarcinoma from the viewpoint of liver lymphatics. J Gastroenterol. 2015;50:913-927. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 56] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 30. | Zhou R, Lu D, Li W, Tan W, Zhu S, Chen X, Min J, Shang C, Chen Y. Is lymph node dissection necessary for resectable intrahepatic cholangiocarcinoma? HPB (Oxford). 2019;21:784-792. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 31. | Chan KM, Tsai CY, Yeh CN, Yeh TS, Lee WC, Jan YY, Chen MF. Characterization of intrahepatic cholangiocarcinoma after curative resection: outcome, prognostic factor, and recurrence. BMC Gastroenterol. 2018;18:180. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 32. | Lin ZY, Liang ZX, Zhuang PL, Chen JW, Cao Y, Yan LX, Yun JP, Xie D, Cai MY. Intrahepatic cholangiocarcinoma prognostic determination using pre-operative serum C-reactive protein levels. BMC Cancer. 2016;16:792. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |